Adiabatic Processes for Monatomic and Diatomic Gases
Here ‘f’ refers to the degrees of freedom of a gas. Monatomic gases have 3 degrees of freedom at room-temperature, whereas diatomic gases have 5. The first three degrees of freedom come from translational degrees of freedom along the x, y, z axes. Diatomic gasses exhibit two extra degrees of freedom because they undergo distinguishable rotations along the θ, ϕ axes. This results in some interesting phenomena conveyed in the following diagrams. These can be used to ask intriguing questions such as;
- Does it require more work to adiabatically compress a diatomic gas rather than a monatomic one?
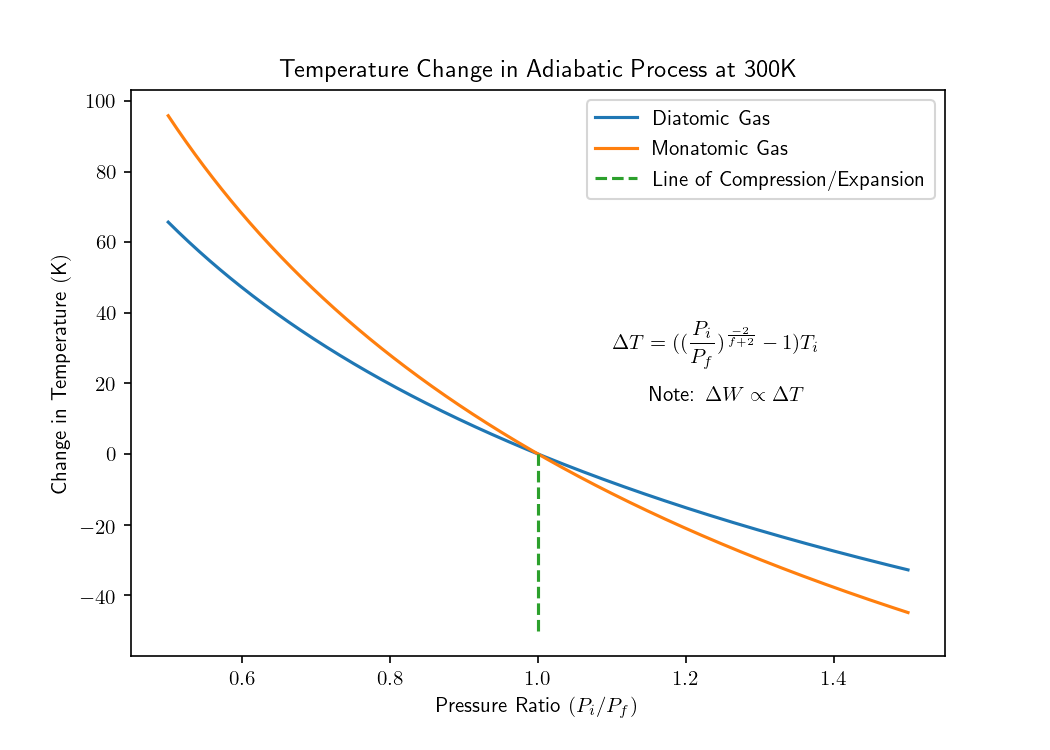
- Given a gas sample is adiabatically compressed, would a diatomic gas have a larger final volume?
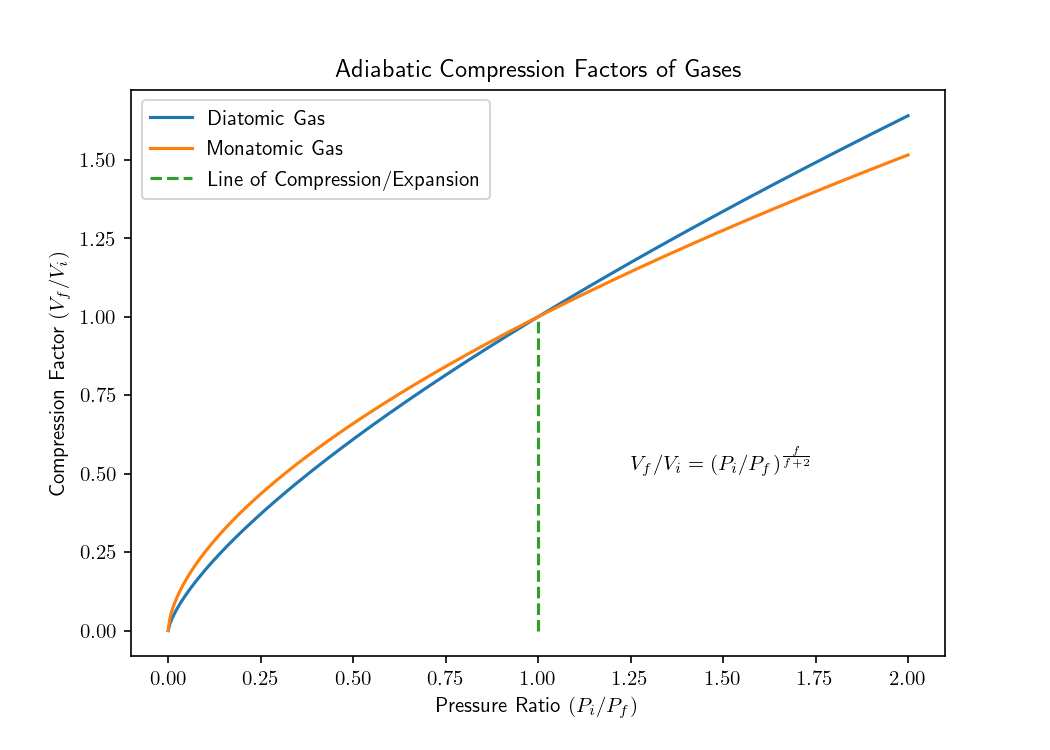
Note that we are considering equi-molar samples of diatomic and monatomic gas.